Fred Hutch Legacy Insiders
Spring 2025
Loyal supporters and their advisors are partnering with Fred Hutch to accelerate life-changing advances in the prevention, detection, and treatment of cancer and infectious disease. We invite you to learn more about the innovation their support makes possible.
Donor spotlight: Renee Hawkes
Taking action to honor a child, a friend and all those experiencing cancer.
 For legacy donor and volunteer leader Renee Hawkes, supporting Fred Hutch combines a commitment to cures with a lifetime of financial expertise.
For legacy donor and volunteer leader Renee Hawkes, supporting Fred Hutch combines a commitment to cures with a lifetime of financial expertise.
“You don’t have to have a life-altering moment to decide it’s time to give to cancer research,” said Hawkes.
But for her, the moment was personal. “My best friend’s daughter, Vivian, was diagnosed with a brain tumor in 2018 at just 4 years old. It was inoperable with no known cure,” she said.
Read more
When Vivian was first diagnosed, Hawkes began looking for a way to take action.
“Watching from the sidelines leaves a hole,” said Hawkes. “I thought, ‘I’ve got to make a bigger difference.”
The research happening at Fred Hutch “was the kind of energy, passion and drive that I was looking for,” she said. In addition to making a personal donation, Hawkes, who is a financial advisor with The Matthews Group at UBS, reached out to the leaders of Fred Hutch’s Professional Advisory Council. The council unites professionals interested in helping Fred Hutch expand charitable giving and assist advisors whose clients may choose to include groundbreaking research in their legacy.
The council was full at the time, but Hawkes didn’t take no for an answer. “I had 45 minutes to pitch why they needed to create one more seat for me,” she said. The pitch was successful, and Hawkes got to work. Now the chair of the council, she continues to encourage her community and clients to support Fred Hutch, whether by giving, volunteering or joining an event.
“Just make that first move, and see how it feels,” she said. “Once you take that step, you start seeing Fred Hutch’s impact everywhere.”
Hawkes also chose to make Fred Hutch a beneficiary of her individual retirement account, or IRA. The choice offered her a way to balance her priorities. “I’m still raising my kids, with one in college,” she said. “So I thought, ‘I can make a bigger impact by sharing what I can leave in the future.” With an IRA, “you can create a beneficiary designation and say, ‘this part goes to my daughters, this part goes to Fred Hutch.’ It creates a legacy for my family and for research.”
When she advises her clients, Hawkes said, they “are thinking about the values reflected in their lives, and those are the values they want to include in their estate plans.” Often, establishing a gift through a will, trust or other planned giving mechanism fulfills that mission.
No matter how people give, Hawkes added that they make a difference. Vivian died two years after her diagnosis, but Hawkes’ support to Fred Hutch remains steadfast.
“Vivian is my ‘why,’” she said. “But since then, I’ve lost others: friends, colleagues and a fellow council member. This is a way we can help. You never know which gift, which dollar, is the one that will uncover the key to cancer. Every single donation and dollar is important.”
— By Laura Anderson
Supporters who contribute to Fred Hutch through a legacy gift become part of our Thomas Legacy Society. To learn more about how you can give through your estate, a donor-advised fund (DAF), an IRA, or in another way, contact us at 206.667.3396 or at plannedgiving@fredhutch.org.
50 years of doing hard things
Founded in 1975 to honor a brother, Fred Hutch Cancer Center pursued bold science, pioneered a cure for blood diseases that changed medicine, and became a world-class biomedical research and clinical care institution

Dr. E. Donnall Thomas, Fred Hutch’s first medical oncology director, consults on rounds during visit with transplant patients.
Tucked away in the Pacific Northwest, Fred Hutch Cancer Center blazed an unusual path when it opened its doors 50 years ago — one of the first eight new comprehensive centers authorized by the 1971 National Cancer Act.
Seattle oncologist and surgeon William Hutchinson, MD, founded the center, which was named in honor of his younger brother, Fred Hutchinson, a Major League Baseball pitcher and manager who died in 1964 of cancer at the age of 45.
Read More
The experimental procedure was difficult to endure and had a miserable track record because of thorny complications that many experts had declared unsolvable. If the skeptics were right and bone marrow transplantation proved to be a bust, that failure might have ushered retreat to less consequential work.
But the Fred Hutch team persisted despite many setbacks. When they finally succeeded, they established a cure for blood diseases that today saves the lives of thousands of patients around the world and launched a new era of medicine that seeks to harness the power of the human immune system.

Baseball legend Joe DiMaggio, left, and Dr. E. Donnall Thomas are greeted by bone marrow transplant patient Darrell Johnson in a LAF (laminar airflow) room, 1978
That victory inspired confidence to try more hard things in the decades that followed, motivated by a simple truth: If we did it once, we can do it again.
In just 50 years, Fred Hutch grew from a regional cancer center into a world-class biomedical research and clinical care institution known for its expertise in molecular biology, tumor virology and infectious diseases, as well as the coordination of large-scale clinical and epidemiologic studies.
Today, Fred Hutch performs leading-edge research and offers clinical care that has evolved from lessons learned solving the hard problem of bone marrow transplant, driven by an enduring commitment to keep doing hard things on behalf of patients and their families.
COURAGE
The same spirit of scientific ambition that motivated the founding of Fred Hutch became manifest in the public imagination more than a decade earlier in 1962 when President John F. Kennedy pledged that the United States would be the first nation to put a human being on the moon.
“We choose to go to the moon in this decade and do the other things, not because they are easy, but because they are hard,” Kennedy said in an address at Rice University in Houston.
Cancer, too, is a hard thing, and as the U.S. embarked on a massive effort toward reaching the moon, the idea that a bone marrow transplant could cure leukemia appeared even further out of reach after many failures.
In 1957, E. Donnall Thomas, MD, who would later become Fred Hutch’s first medical oncology director, published a landmark study in the New England Journal of Medicine about six patients with end-stage leukemia he had treated with radiation, chemotherapy and an intravenous infusion of bone marrow. The grafts established in only two patients and all six had died by 100 days after the transplant.
But Thomas showed for the first time that human bone marrow could be collected, stored and given by intravenous injection without causing harm.
In 1960, Thomas found his first success in Seattle: a 6-year-old girl with aplastic anemia, which was fatal at the time with no treatments. Thomas helped perform a transplant with bone marrow donated from the girl’s identical twin, making her likely the first patient ever cured with a bone marrow transplant.
Making the procedure work with bone marrow donors who weren’t identical twins proved much harder. Prominent immunologists argued that it was doomed to fail because they believed the very biology of the human immune system would conspire against transplanted marrow at every turn.
But Thomas didn’t give up, instead turning to experiments with animals to better understand what he was up against.
The same year Kennedy promised we would reach the moon, Thomas reported in the journal Blood that five out of 41 animals lived beyond four months after receiving bone marrow transplants when they were given an anti-inflammatory drug, methotrexate, which helped the transplanted tissue get established.
In 1963, Thomas moved his lab to Seattle from Cooperstown, New York and spent the rest of the decade honing the technique, publishing several studies demonstrating its potential.
Six years later as astronaut Neil Armstrong kept Kennedy’s promise and walked on the moon, Thomas initiated a clinical trial program in Seattle for bone marrow transplants in humans.
But working in a public health hospital slated for closure, Thomas needed a permanent home for his quest.
Meanwhile, Dr. Hutchinson needed a director of clinical research for the new cancer center that would emerge from the research foundation Hutchinson established in 1956 — the first private, nonprofit, biomedical research institute in the Pacific Northwest.
Hutchinson and Thomas’s partnership became official in 1975 when Fred Hutchinson Cancer Research Center opened in Seattle’s First Hill neighborhood.
The same year, Thomas published research showing that he had cured a minority of patients rescued in leukemia’s final stages.
Four years later, he published results showing a cure rate of more than 50% for patients who received transplants at an earlier stage when their leukemia was in remission.
In less than two decades since his landmark study, bone marrow transplant became an accepted therapy that changed the course of clinical medicine.
In 1990, Thomas was awarded the Nobel prize in physiology or medicine, the first of three Nobels won by Fred Hutch researchers.
Over the next 20 years, more than a million hematopoietic stem cell transplants would be performed and as of 2024, more than 1.5 million have been performed at more than 1,500 transplantation centers around the world.
The courage to pursue bone marrow transplantation and achieve positive results gave Fred Hutch researchers the confidence to do more hard things.
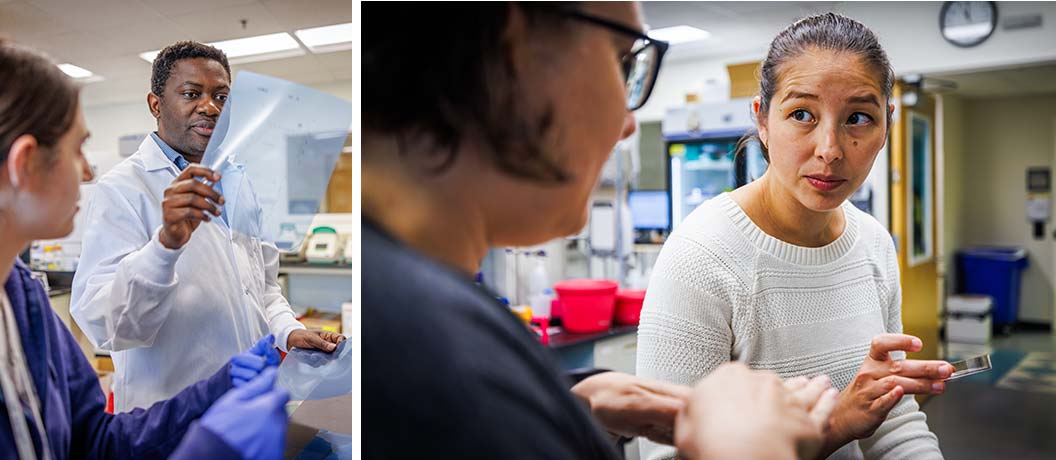
Researchers in Basic Sciences Division, Drs. Richard Adeyemi, and Melody Campbell (right)
CONFIDENCE
While the clinical team solved the hard problem of bone marrow transplant, Fred Hutch pursued Hutchinson’s other two missions: providing local physicians and researchers scientific expertise in fundamental biology and population-based studies of disease.
From the start, Fred Hutch recruited scientists in fields relevant to cancer, but especially sought experts in molecular biology and the role of viruses in tumor formation, which were underrepresented fields of research in the Seattle area.
Founded at the onset of Fred Hutch’s transition to a divisional structure in late 1981, the Basic Sciences Division brought together scientists seeking to understand the fundamental mechanisms of life and how the molecular processes that govern it can go awry, causing diseases such as cancer — discoveries that underly the innovative cures and therapies developed worldwide and at Fred Hutch.
Paul Neiman, MD, PhD, a member of Thomas’s original bone marrow transplant team, was the division’s first director. He and the division’s founding members established a scientific culture driven by egalitarianism, collaboration, inclusivity and creativity.
They agreed that faculty would collectively select and hire new faculty and investigators would run their own small labs, working primarily at the bench, mentoring the next generation of scientists rather than sequestered in an office administering big labs from afar.
Such an approach encourages investigators to step out of their comfort zones and pursue creative ideas they might otherwise have considered too risky for their careers.
And they collaborate early and often within Fred Hutch and with other research institutions, near and far, to augment resources and include different perspectives and areas of expertise to generate new scientific insights.
These values ensure a confident culture of scientific ambition that emphasizes the quality of publications over quantity and favors big ideas unconstrained by an institutional fear of failure.
That culture has produced many breakthroughs such as identifying the atomic structures of proteins, understanding the molecular details of gene regulation and principles of development in organisms from embryo to adult.

Women Health Initiative group photo, 2023
The Public Health Sciences Division also traces its roots to the foundation of Fred Hutch when it was called the Program in Epidemiology and Biostatistics and became the home for the first cancer prevention and control unit in the country funded by the National Cancer Institute in 1983.
Under the leadership of biostatistician Ross Prentice, PhD, the division served as a premier hub for research in biostatistics, cancer prevention and epidemiology, hosting many national data and clinical coordination centers including the Women’s Health Initiative, which has provided data for more than 2,000 scientific papers and made critical discoveries regarding the relationship between hormones and breast cancer.
Fred Hutch also recruited experts who beat a well-worn path between patient’s bedsides and laboratory workbenches to solve the problem of bone marrow transplantation.
Transplant patients, for example, were unusually susceptible to infectious disease, so Fred Hutch hired an expert from the Centers for Disease Control and Prevention.
What began as a one-person research program to solve that problem — combined with the desire to expand Fred Hutch’s expertise in how viruses and pathogens cause cancer — evolved into the Vaccine and Infectious Disease Division, one of the largest assemblies of infectious disease researchers at any cancer center.
Fred Hutch pioneered and continues to lead research into the role of cancer-causing viruses. Fred Hutch research on HPV (human papillomaviruses) in cervical cancer paved the way for the HPV vaccine, which has the potential to eliminate over 95% of all cervical cancers.
And Fred Hutch leads the world’s largest publicly funded collaboration focused on development of vaccines to prevent HIV/AIDs, with a clinical trials infrastructure to carry out the research, which made Fred Hutch a natural choice to coordinate development of a vaccine for the virus that causes COVID-19.
Fred Hutch also leads research to find better treatments, vaccines and cures that target HSV, which is responsible for cold sores and genital herpes, and other members of the herpesvirus family. These viruses are especially dangerous for people whose immune systems are compromised by HIV/AIDS or who are recovering from bone marrow or blood stem cell transplants.
Over the years, Fred Hutch has expanded to seven research divisions — including Human Biology, Translational Science and Therapeutics and Radiation Oncology — to leverage scientific discoveries into safe and effective therapies to prevent, treat and cure cancer and infectious diseases.
Several integrated research centers, institutes, networks and other cross-divisional programs have earned Fred Hutch an international reputation for collaboration. HICOR, the Hutchinson Institute for Cancer Outcomes Research, for example, leads research aimed at reducing the economic and human burden of cancer and other health disparities.
Fred Hutch also leads the way on prostate cancer research as the headquarters for an international collaboration that brings together forward-thinking prostate cancer researchers at Fred Hutch, the University of Washington, Oregon Health & Science University and the University of British Columbia.
Work by Fred Hutch scientists led to the discovery that inherited mutations in the BRCA1/2 genes can drive not just breast and ovarian cancers, but also cancers of the prostate.
Another hard thing on the horizon for cancer research is making sense of the mountains of biological data researchers can extract from patient genetics to better understand exactly what’s making them sick and what specifically could make them better.
It’s become known as “precision oncology” — the practice of individualizing a person’s cancer screening, prevention and treatment strategies to help personalize their care and improve their outcomes.
Here, too, Fred Hutch is poised to lead the way through the Stuart and Molly Sloan Precision Oncology Institute.

HICOR leaders Drs. Veena Shankaran (left) and Scott Ramsey (right)
COMMITMENT
Fred Hutch began with a single-minded focus on applying laboratory research to help a small group of patients considered incurable.
When it was founded, Fred Hutch had just a 20-patient specialized unit dedicated to terminal blood cancer treatment.
In the 1970s, other cancer centers that had started at the same time broadened their reach to cover a wide range of tumors, emphasizing the word “comprehensive” in their national designation.
As it grew over the decades, Fred Hutch focused on improving bone marrow transplantation procedures as well as other promising therapies and collaborated with other institutions that provided cancer care.
In 2022, Fred Hutchinson Cancer Research Center merged with Seattle Cancer Care Alliance and restructured its relationship with UW Medicine, integrating clinical patient care. The newly branded Fred Hutch Cancer Center reaffirmed its commitment to “compassionate, connected patient care that puts the patient and family at front and center.” Fred Hutch now has eight clinical locations in the Puget Sound region.
Today, Fred Hutch is well on the way to turning Thomas’ key insight — that the immune system can be harnessed to eradicate cancer — into new therapies at the leading edge of a new field pioneered in Seattle: immunotherapy.
By understanding the role of disease-fighting immune cells called T cells in bone marrow transplants, Fred Hutch researchers spent decades learning how to manipulate them to target a wide range of cancers and HIV without harming healthy cells.
Some of those therapies genetically engineer a patient’s own T cells to recognize and fight cancer, one of the first examples of precision oncology. Others steer radiation to cancer cells or boost immune responses that cancer hijacks to evade the body’s natural defenses.
Fred Hutch also is developing other cancer vaccines to prevent cancer, much like the HPV vaccine, or trigger an immune response to attack the disease.
The courage to do one hard thing — bone marrow transplantation — made this new era of cellular immunotherapy possible and may ultimately make those transplants rarely needed.
Courage, confidence and commitment to do hard things defined the first half century of Fred Hutch and those virtues light the path forward.
John Higgins, a staff writer at Fred Hutch Cancer Center, was an education reporter at The Seattle Times and the Akron Beacon Journal. He was a Knight Science Journalism Fellow at MIT, where he studied the emerging science of teaching. Reach him at jhiggin2@fredhutch.orgor @jhigginswriter.bsky.social
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org
Prostate cancer research: Collaborating on groundbreaking discoveries
Fred Hutch and UW Medicine researchers unite to pioneer early detection, precise treatment, and prevention strategies
Fred Hutch 2024 Annual Report
Gaining Ground on Our Future.
A recap of the past year at Fred Hutch. Read about significant developments and highlights from our research, clinical care, philanthropy and people.
Two doses of the HPV vaccine is all you need
Long-term immunity from human papillomavirus infections — and the deadly cancers they can drive — reached with just two jabs
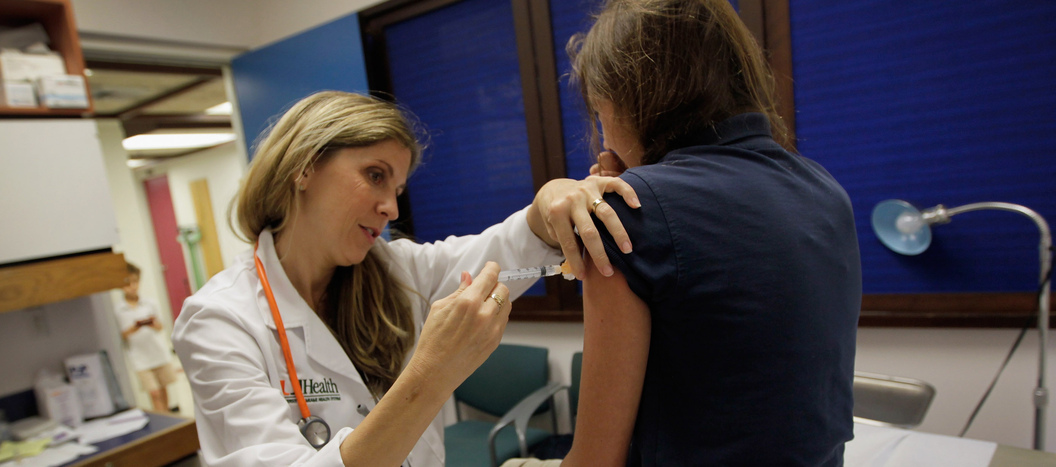
Fred Hutch’s Dr. Denise Galloway was the first to discover that HPV, the human papilloma virus, had the potential to drive cancers. “In just 25 years, we went from not having any idea what viruses were involved in these cancers to having a vaccine,” she said. “That’s amazingly fast.” The vaccines are regularly given to pre-teens, as shown here, to protect them from HPV and the various cancers they can cause.
A study led by Fred Hutch Cancer Center cancer biologist Denise Galloway, PhD, that looked at the staying power of the HPV vaccine has found that two doses provide the same amount of immune protection as three, with no additional boosters needed.
“We looked at long-term immunity, or immunogenicity — the ability to trigger an immune response — using two separate studies with kids who were vaccinated at 10 to 12 years old and then a subset that were invited back either three or almost 10 years later,” said Galloway, whose research decades ago was instrumental in linking human papillomavirus, or HPV, infections to several cancers and in designing trials for the HPV vaccine.
“We looked for memory B cells as a marker that you had a vaccine and your immune system remembers it,” she said. “The answer was yes, and it was the same for either three or two doses.”
Read More
What does this mean for the future? Galloway said it’s the first step to simplifying HPV-related cancer prevention even more.
“Two doses is the standard now in kids under 15 in the U.S.,” she said. “Now we’re interested in getting it down to one dose.”

“The U.S. saves about $8 billion a year by preventing these cancers,” said Fred Hutch molecular biologist Dr. Denise Galloway, shown here with Fred Hutch president and director Dr. Thomas Lynch, Jr., receiving the Discovery Science Award during the 2024 Fred Hutch Faculty Conclave.
Study’s global significance is huge
Developing a single-dose HPV vaccine will have a large impact globally, said Galloway, who serves as the scientific director of Fred Hutch’s Pathogen-Associated Malignancies Integrated Research Center and holds the Paul Stephanus Memorial Endowed Chair.
“It’s going to make a big difference in the developing world where you’re lucky to get one dose,” she said. “Globally, it’s very hard to follow people and it’s expensive to have more than one dose. That’s where it will have the biggest effect.”
But in the U.S., it will also go far to help to protect people against HPV infections.
HPV infections, transmitted through skin-to-skin contact, are more or less ubiquitous among adults across the globe. Most HPV infections clear up on their own, but can morph into precancers that ultimately cause cervical, anal, vulvar, penile and head and neck cancers.
“A single dose will make it easier here in the U.S.,” Galloway said. “You won’t have to think about when you come back for the next dose. Just do it once and get it over with.”
First approved by the FDA in 2006, the HPV vaccine has been administered to millions of young women and men around the world.
In Scotland, where the vaccine is provided free to school children, cervical cancer rates have plummeted. Australia, too, has a highly successful HPV vaccination program and according to the newsletter HPV World, is on track to completely eradicate cervical cancer.
“The U.S. saves about $8 billion a year by preventing these cancers,” Galloway said. “Although not everybody is getting the vaccine. The International Papillomavirus Society thinks that we can eliminate cervical cancer in this country with the vaccine and screening, which would be very cool.”
The HPV vaccine prevents new infections and is most effective when given to children before they’re sexually active.
Screening and valid information still key
But even after receiving the HPV vaccination, people should continue to be screened, Galloway said.
“One of the good things is these cancers move slowly, so if you’re screened regularly, you should catch it,” she said. “Although some people don’t go in for screening, or they can’t afford to go.”
Every year, around 11,500 new cases of cervical cancer are diagnosed in the U.S. and about 4,000 women die of the disease. Almost all of these cases are driven by an HPV infection, which has no symptoms.
When caught and treated early, cervical cancer is highly survivable and death rates for the disease have dropped by more than half since the mid-1970s. According to the American Cancer Society, this is a direct result of prevention and screening (usually via pap smear or HPV test). Research shows that in states with expanded Medicaid programs, more people get cervical cancer screenings. Cervical cancer death rates for Black and Native American women are still about 65% higher than they are for white women.
The HPV vaccine has gone through “more than 160 studies that show [they] have a favorable safety profile — the body of scientific evidence overwhelmingly supports their safety,” per the Center for Disease Control and Prevention, or CDC.
Still, misinformation surrounding HPV vaccination has been rampant — and profitable, per a 2024 study of Instagram influencers done by the University of Washington. One study that dug into comments about HPV vaccination on social media found misinformation regarding “adverse reactions, unnecessary vaccines, conspiracy theories, and mistrust of authority,” with Facebook publishing the highest proportion of misinformation in its comments.
HPV vaccine information and resources
- Currently, the CDC recommends HPV vaccines for children at around age 11 or 12, although kids as young as age 9 can be vaccinated.
- Additionally, adults up to age 26 can be vaccinated if they haven’t received one previously. Most health insurance covers the cost of the vaccine.
- You can get HPV vaccines at the doctor’s office, at pharmacies, state health departments, community health clinics and at some schools. The Washington State Childhood Vaccine Program provides the HPV vaccine at no cost to adolescents less than 19 years of age.
- Cervical, breast and colorectal cancer screenings are free for eligible Washington state residents through the Breast, Cervical and Colon Health Program.
One and done?
Now that Galloway and her team have determined that two or three jabs guarantees the immune system will remember the virus for at least 10 years, she said she wants to study the long-term immunity, or immunogenicity, of just one dose.
“Millions of women have taken the vaccine, and these cancers are being prevented,” she said. “If you look at the rates of cervical cancer lesions in young women who’ve been vaccinated, they’ve gone down a lot.”
More than 200 distinct types make up the HPV virus family with HPV 16 and HPV 18 causing about 70% of cervical cancers as well as a higher percentage of both head and neck and anal cancers.
Current HPV vaccines pack a punch, protecting against these detrimental types and others, along with genital warts. But as with all discoveries, Galloway believes there’s always room for improvement.
”I’m excited to see if one dose will really hold up over the long run,” she said.
This study was partially funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA.
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org.
Diane Mapes is a senior editor and writer at Fred Hutch Cancer Center, who’s written for NBC News, TODAY, CNN, MSN, Seattle Magazine and other publications. A breast cancer survivor and patient advocate, you can reach her at dmapes@fredhutch.org or doublewhammied.com / @double_whammied / @doublewhammied.bsky.social. Just diagnosed and need information and resources? Visit our Patient Care page.
New methods reveal cancer mechanism in ancient genes
Fred Hutch researchers discover that overproduction of DNA packaging material predicts aggressive brain and breast tumors, which could lead to cheaper diagnostic tests and new drug therapies

Fred Hutch microbiologist Steven Henikoff prepares paraffin-embedded slides in lab.
Researchers at Fred Hutch Cancer Center have discovered an overlooked mechanism driving aggressive breast and brain tumors involving genes so ancient — more than 2 billion years old — that they fly under the radar of standard genetic sequencing methods.
“Identifying this mechanism suggests it could be a new test to diagnose cancers and possibly treat them,” said Fred Hutch molecular biologist Steven Henikoff, PhD, co-first author of a study recently published in the journal Science.
The study focuses on 64 ancestral genes needed to make histones, which are the molecular packaging material that helps squish some six feet of DNA strands into a single cell’s nucleus.
Read more
The discovery reveals a new biomarker to aid in the early detection of disease and a potential target for more precisely tailored therapies — made possible by a collaboration between labs in different research divisions that defines the scientific culture Fred Hutch has nurtured for half a century.
Making the most from patient tumor samples
The histone study began with a phone call between Henikoff in the Basic Sciences Division and co-author Eric Holland, MD, PhD, a Fred Hutch brain cancer researcher who heads the Human Biology Division and holds the Endowed Chair in Cancer Biology.
Part of Fred Hutch’s commitment to building preeminence in precision oncology — diagnosis and treatment tailored to a patient’s individual biology — involves research that requires extracting molecular information from preserved patient tumor samples.
Cross-referencing that molecular information with patient medical records enables researchers to match genetic profiles to clinical outcomes and classify tumors based on their biology, which is more accurate than simply lumping them together based on how they look under a microscope.
Though all human cells share the same DNA, each kind of cell — such as brain, skin and kidney — requires different genes to be turned on or expressed at different times depending on that cell’s function. Many things can go wrong in that process that may turn cells cancerous.
Gene expression begins in the cell’s nucleus with transcription, which makes RNA copies of genetic sequences from DNA, which serve as templates. Those copies are delivered in the form of RNA molecules to factories in the cell that make proteins, the cell’s molecular workers. The factory reads the RNA template, translating the genetic sequences into the amino acid sequences needed to make each protein.
Holland explains it like this: Imagine that a cell’s nucleus is like the Library of Congress.
The cell’s DNA, packaged into chromosomes inside the nucleus, contains all the library’s books accumulated over the cell’s long evolution on Earth. Those books can never be checked out.
But they can be copied, and those copies can be carried out of the library by RNA molecules.
A standard method of mining samples for molecular information is called RNA sequencing, which tells you what books in the library are getting copied a lot based on how many copies are in circulation outside the library.
From that information, you can figure out which genes got copied inside the nucleus and how often, which reveals patterns of gene expression in healthy cells that may change in cancerous ones.
RNA sequencing works best on fresh-frozen cells, but that’s not usually how samples from surgery or biopsies are prepared.
For more than a century, the preferred method for long-term preservation of samples involves fixing fresh tissue in an embalming fluid called formalin and embedding the tissue in paraffin wax. That process creates formalin-fixed paraffin-embedded (FFPE) samples, the most common kind available for research.
Like most hospitals and research institutions, Fred Hutch has plenty of these samples spanning decades, but long-term exposure to the formalin damages the genetic material, making those samples practically unreadable with standard RNA sequencing.
When Henikoff learned that Holland wanted to find ways to mine more molecular information from their samples, he saw an opportunity for the two labs to collaborate.
“I called him up and asked if he was just going to use RNA sequencing, because we have something that might work pretty well,” Henikoff said.
He remembered Holland joking that he had so many paraffin blocks around the lab he used them as doorstops.
“I decided he’d be fun to work with,” Henikoff said.
Henikoff had developed a faster, cheaper alternative to RNA sequencing that he had tweaked to get molecular information out of paraffin-embedded samples.
Henikoff’s sequencing method is called Cleavage Under Targeted Accessible Chromatin, or CUTAC.
It’s different than standard RNA sequencing because it reveals what is going on at the beginning of the transcription process when DNA is getting copied inside the nucleus rather than at the end when RNA copies already are in circulation.
CUTAC identifies which books are getting copied as it’s happening.
It does this by tracking the activity of an enzyme called RNA polymerase II as it moves across DNA, pausing at various places where it accumulates and kick-starts the copying process, including stretches concerning gene regulation that don’t get picked up by RNA sequencing.
Using CUTAC, the Henikoff and Holland labs were able to distinguish different differences in gene regulation between tumor and normal tissues in mouse brain and liver samples.
“This is a very inexpensive way to get a lot of information out of paraffin sections, and Steve’s lab developed it,” Holland said.
They published the results in Nature Communications in 2023.
The next step was to apply CUTAC to multiple cancer types, which led to the current study.
Finding an ancient mechanism
Henikoff and his team used the modified CUTAC method to better understand a common phenomenon in cancer called hypertranscription, which predicts a poor prognosis.
It’s characterized by an overall increase in RNA polymerase II, which turns on thousands of genes, keeping the library’s copy machines in the nucleus working overtime.
But hypertranscription expresses so many genes at the same time that it’s difficult to isolate the mechanism that drives aggressive tumors.
Henikoff figured the most relevant genes to study would be the ones that limit how fast cells can double.
He zeroed in on a subset of just 64 ancestral genes needed to make histones, which are the molecular packaging material that helps squish DNA inside the cell’s nucleus.
DNA strands wrap around histones, which are clustered into eight-histone balls that are threaded like beads on a string into fibers that are further intertwined to form chromosomes.
Cells don’t store extra histones like cardboard boxes in the garage.
On moving day when cells replicate their DNA and divide, histones must be rapidly produced just in time for the cell to copy its chromosomes and make sure each new cell gets a complete and identical set.
When that happens, there’s such an abundance of RNA polymerase II lingering over histone genes in mice and fruit flies during cell replication that levels of the enzyme drop by as much as 40 percent elsewhere.
But the potential role of histones in fueling cancer growth has been overlooked in the field because they fly under the radar of standard RNA sequencing.
When most genes are copied, the RNA molecules carrying the copies to the protein factories acquire a stabilizing modification that gives them a distinct chemical signature, making them detectable by RNA sequencing.
But histone genes are so old they predate the rise of eukaryotic life (organisms with cells that contain a nucleus, which emerged between 1.8 and 2.7 billion years ago).
Their RNA copies use more ancient chemistry to stabilize, and because they lack that common signature, they’re like a plane that’s invisible to the control tower because it’s flying without a transponder.
Henikoff’s CUTAC method solves that problem because it tracks which DNA is getting copied at the beginning of the transcription process inside the nucleus instead of the resulting RNA copies at the end of the process.
They hypothesized that the single functional role of hypertranscription in cancer is to crank out enough histones to keep pace with the DNA packaging requirements for tumor cells to proliferate faster than normal.
They tested their hypothesis on a set of 36 human meningioma brain tumor samples from Holland’s lab, which are cross-referenced with patients’ medical histories.
That enabled them to link tumor biology with patient outcomes using paraffin-embedded samples already collected and stored.
They discovered that overproduction of histones alone predicted the aggressiveness and recurrence of meningioma tumors. Overproduction of histones also predicted aggressiveness of invasive breast cancer based on an analysis of 13 paraffin-embedded samples.
One of the study’s co-first authors, Ye Zheng, PhD, then a postdoctoral researcher in Henikoff’s lab, discovered a correlation between elevated histone levels and changes to chromosomes that drive many, but not all cancers.
When cells divide, each of the 46 chromosomes makes a copy of itself, and the copies are hinged by a structure called a centromere that divides the pair into four arms.
Zheng, who is now an assistant professor of Bioinformatics and Computational Biology at the University of Texas MD Anderson Cancer Center, found that overproduction of histones is positively correlated with the whole-arm chromosome losses in brain and breast cancer.
The finding is consistent with other research linking the overproduction of histones with breaks to the centromere hinges that are essential to making sure each new cell gets a complete set of chromosomes.
Though histone genes comprise only 1/100,000th of the human genome, the over-expression of this tiny, ancient subset of DNA — all by itself — predicted poor outcomes in brain and breast cancers, making it a potentially powerful new biomarker for disease with the potential to improve diagnosis, prognosis and even new therapies.
This all began with a phone call. It’s the kind of thing that’s perfect for Fred Hutch. We are integrated. We know one another. It’s like one family.
— Steven Henikoff
Making precision oncology more precise
The team analyzed paraffin-embedded meningioma samples from a collection Holland and his colleagues have amassed to build the field’s largest meningioma dataset to date, comprising nearly 1,300 tumors sampled from patients all over the world combined with detailed clinical treatment histories for many of those cases.
Most of those sample are fresh-frozen in liquid nitrogen, the preferred preservation method for RNA sequencing.
Using computational tools invented at Fred Hutch, Holland’s lab simplified that information, which comprises millions of data points, and represented it graphically on a digital reference map for meningioma tumors.
The map revealed a complex landscape of seven general regions of tumors, further divided into several distinct subtypes based on similar genetics, tumor severity and treatment outcomes.
Zheng worked some computational wizardry to harmonize the CUTAC-derived data with the RNA sequencing data to fit the paraffin-embedded samples into the larger reference map.
To her surprise, the two data sets fit well together, which means that a paraffin-embedded sample sequenced with CUTAC can be plopped down on a map of RNA-sequenced tumors near the ones they most closely resemble on a molecular level.
The integration was especially striking when they had samples from the same patient using both methods. The sample analyzed with CUTAC and the one analyzed with RNA sequencing landed on Holland’s map close to one another.
“We can not only cluster them close together, but they also almost have an overlapping pattern,” Zheng said.
That tight integration means that patients whose tumors are analyzed using the faster, cheaper CUTAC method can still benefit from all the data derived from other patients with similar tumors — the nearest neighbors on the map — that were analyzed with RNA sequencing.
“You can gain all the information from the reference landscape: clinical data, prediction of outcome and response to therapy,” Holland said.
The collaboration between labs opened new avenues for better diagnosis and potential new therapies, but it also provided a wealth of previously inaccessible samples that will help Henikoff and his colleagues better understand the fundamental processes of histone overproduction that contribute to cancer.
“This all began with a phone call,” Henikoff said. “It’s the kind of thing that’s perfect for Fred Hutch. We are integrated. We know one another. It’s like one family.”
This work was supported by the Howard Hughes Medical Institute and grants from the National Institutes of Health and National Cancer Institute.
John Higgins, a staff writer at Fred Hutch Cancer Center, was an education reporter at The Seattle Times and the Akron Beacon Journal. He was a Knight Science Journalism Fellow at MIT, where he studied the emerging science of teaching. Reach him at jhiggin2@fredhutch.org or @jhigginswriter.bsky.social.
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org.
Join the Thomas Legacy Society
The Thomas Legacy Society at Fred Hutch is a special group of supporters who have made a commitment through a legacy gift to drive lifesaving cancer research and improve the lives of patients in the years to come.
What does a unique gift like this actually do? By making a gift to Fred Hutch in your will or trust, or by naming us as beneficiary on one of your financial or investment accounts, you join a community fueling innovation by:
- Funding early-stage ideas that push the boundaries of science and are not yet eligible for federal funding.
- Discovering new ways to prevent diseases from ever occurring.
- Supporting early-career investigators who are working on the cures of the future.
- Investing in technology and equipment that accelerate discovery.
- Expanding support services for patients and caregivers.
Visit our website to learn more or contact us.
Have you already included Fred Hutch in your estate plans? Please let us know so we can ensure your wishes are carried out as you intend!

