Fred Hutch Legacy Insiders
Winter 2025
Loyal supporters and their advisors are partnering with Fred Hutch to accelerate life-changing advances in the prevention, detection, and treatment of cancer and infectious disease. We invite you to learn more about the innovation their support makes possible.
Donor spotlight: Renee Hawkes
Taking action to honor a child, a friend and all those experiencing cancer.
 For legacy donor and volunteer leader Renee Hawkes, supporting Fred Hutch combines a commitment to cures with a lifetime of financial expertise.
For legacy donor and volunteer leader Renee Hawkes, supporting Fred Hutch combines a commitment to cures with a lifetime of financial expertise.
“You don’t have to have a life-altering moment to decide it’s time to give to cancer research,” said Hawkes.
But for her, the moment was personal. “My best friend’s daughter, Vivian, was diagnosed with a brain tumor in 2018 at just 4 years old. It was inoperable with no known cure,” she said.
Read more
When Vivian was first diagnosed, Hawkes began looking for a way to take action.
“Watching from the sidelines leaves a hole,” said Hawkes. “I thought, ‘I’ve got to make a bigger difference.”
The research happening at Fred Hutch “was the kind of energy, passion and drive that I was looking for,” she said. In addition to making a personal donation, Hawkes, who is a financial advisor with The Matthews Group at UBS, reached out to the leaders of Fred Hutch’s Professional Advisory Council. The council unites professionals interested in helping Fred Hutch expand charitable giving and assist advisors whose clients may choose to include groundbreaking research in their legacy.
The council was full at the time, but Hawkes didn’t take no for an answer. “I had 45 minutes to pitch why they needed to create one more seat for me,” she said. The pitch was successful, and Hawkes got to work. Now the chair of the council, she continues to encourage her community and clients to support Fred Hutch, whether by giving, volunteering or joining an event.
“Just make that first move, and see how it feels,” she said. “Once you take that step, you start seeing Fred Hutch’s impact everywhere.”
Hawkes also chose to make Fred Hutch a beneficiary of her individual retirement account, or IRA. The choice offered her a way to balance her priorities. “I’m still raising my kids, with one in college,” she said. “So I thought, ‘I can make a bigger impact by sharing what I can leave in the future.” With an IRA, “you can create a beneficiary designation and say, ‘this part goes to my daughters, this part goes to Fred Hutch.’ It creates a legacy for my family and for research.”
When she advises her clients, Hawkes said, they “are thinking about the values reflected in their lives, and those are the values they want to include in their estate plans.” Often, establishing a gift through a will, trust or other planned giving mechanism fulfills that mission.
No matter how people give, Hawkes added that they make a difference. Vivian died two years after her diagnosis, but Hawkes’ support to Fred Hutch remains steadfast.
“Vivian is my ‘why,’” she said. “But since then, I’ve lost others: friends, colleagues and a fellow council member. This is a way we can help. You never know which gift, which dollar, is the one that will uncover the key to cancer. Every single donation and dollar is important.”
— By Laura Anderson
Supporters who contribute to Fred Hutch through a legacy gift become part of our Thomas Legacy Society. To learn more about how you can give through your estate, a donor-advised fund (DAF), an IRA, or in another way, contact us at 206.667.3396 or at plannedgiving@fredhutch.org.
Fred Hutch 2024 Annual Report
Gaining Ground on Our Future.
A recap of the past year at Fred Hutch. Read about significant developments and highlights from our research, clinical care, philanthropy and people.
Prostate cancer research: Collaborating on groundbreaking discoveries
Fred Hutch and UW Medicine researchers unite to pioneer early detection, precise treatment, and prevention strategies
For Fred Hutch biostatisticians, numbers are no game
Meticulous work at the SWOG Statistics and Data Management Center, housed at Fred Hutch, may alter care for bladder cancer and lymphoma
 Two potentially practice-changing studies published this month in the New England Journal of Medicine showcase the biostatistical expertise at the SWOG Statistics and Data Management Center (SDMC) housed at Fred Hutch Cancer Center. One upends the common approach at many high-volume institutions of removing more, rather than fewer, lymph nodes during surgery for muscle-invasive bladder cancer. The other yielded promising results for the combination nivolumab (a targeted therapy drug) plus AVD chemotherapy for stage 3 or 4 classic Hodgkin lymphoma.
Two potentially practice-changing studies published this month in the New England Journal of Medicine showcase the biostatistical expertise at the SWOG Statistics and Data Management Center (SDMC) housed at Fred Hutch Cancer Center. One upends the common approach at many high-volume institutions of removing more, rather than fewer, lymph nodes during surgery for muscle-invasive bladder cancer. The other yielded promising results for the combination nivolumab (a targeted therapy drug) plus AVD chemotherapy for stage 3 or 4 classic Hodgkin lymphoma.
Read More
This year marks the 40th anniversary of the SDMC moving to Seattle from Houston after Fred Hutch won the National Cancer Institute (NCI) grant that provides much of the center’s funding. John J. Crowley, PhD, was Fred Hutch’s original SDMC group statistician and director, roles he held until 2012.
Behind-the-scenes experts are involved from start to finish
SWOG Cancer Research Network is one of five cooperative groups in the United States that make up NCI’s National Clinical Trials Network. More than 1,300 institutions and 20,000 individual members are part of SWOG. Like each group in the clinical trials network, SWOG has a statistics and data management center, co-located at Fred Hutch and Cancer Research and Biostatistics (CRAB), a nonprofit in Seattle, and a network operations center, based at Knight Cancer Institute at Oregon Health & Science University. CRAB Chief Executive Officer Antje Hoering, PhD, is a Fred Hutch affiliate professor.
All SWOG studies go through the hands of LeBlanc, Tangen and other SDMC staff at Fred Hutch and CRAB. These experts contribute to study design, review and quality assurance; collect and manage data; randomize study participants; produce reports; analyze and interpret data; provide data and safety monitoring; prepare manuscripts; and develop, maintain and support computing systems. At any given time, SWOG has about 100 studies that are in active development, are enrolling patients or have closed enrollment but are following patients.
“The SDMC has stayed here for four decades in part because Fred Hutch is Fred Hutch: it’s historically strong in both biostatistics and cancer prevention, both interests of SWOG. That’s part of the long-term success,” said LeBlanc.
To ensure care is evidence-based, first collect the evidence
Tangen is a co-author on the phase 3 study comparing standard versus extended lymphadenectomy, the surgical removal of lymph nodes to check for cancer spread, in people with bladder cancer that has invaded the bladder’s muscle wall. Though Fred Hutch clinical investigators typically enroll patients in SWOG studies, they didn’t for this particular trial.
“At the Statistics and Data Management Center, we’re regularly interacting with researchers from around the country, but not necessarily with the people down the hallway,” who may be focused on some of the many other types of research also taking place at Fred Hutch, said Tangen. Most SDMC-associated faculty members and several SDMC staff do collaborate with other Fred Hutch faculty on research that’s non-SWOG related.
 The study randomized nearly 600 patients with localized muscle-invasive bladder cancer to receive either extended or standard lymphadenectomy along with bladder removal. Three dozen experienced urologic oncology surgeons at 27 sites were rigorously credentialed to adhere to the study protocol, reducing differences in care that might have clouded the results.
The study randomized nearly 600 patients with localized muscle-invasive bladder cancer to receive either extended or standard lymphadenectomy along with bladder removal. Three dozen experienced urologic oncology surgeons at 27 sites were rigorously credentialed to adhere to the study protocol, reducing differences in care that might have clouded the results.
Many academic medical centers and other high-volume centers have adopted more extensive lymph node removal as the standard of care, though there was only low-level evidence showing this was better for patients, according to the published report.
The research team set out to test the hypothesis that a more aggressive approach improves disease-free and overall survival. In fact, they found extensive lymphadenectomy doesn’t provide disease-free or overall survival benefit, and it’s linked with higher rates of health problems or death around the time of surgery (perioperative morbidity and mortality).
Reliable results require careful design
“The most important part of the biostatistician’s role is to be involved at the early stages of designing a study in order to get convincing results at the end. It might not be the result the researchers wanted, but it will be a reliable answer,” said Tangen.
For example, for this study, the early steps included Tangen talking with principal investigator Seth P. Lerner, MD, of Baylor College of Medicine Medical Center, in 2009, to define his study question. Next, she got to work coming up with a sample size and power calculations — essentially determining how many patients would need to enroll to achieve the number of events (such as relapse or death) that would allow the team to answer the primary objective. The biostatistics team also designed tools for collecting surgical quality control and complication data.
“This can be an iterative process between the biostatisticians and others involved in designing the trial, to come up with a study that’s both scientifically meaningful and feasible to run,” said Tangen. “You need a clear road map and then have to follow it.”
Which is better? Comparing medicine combinations
The study on classic Hodgkin lymphoma compared outcomes for adolescents and adults with newly diagnosed stage 3 or 4 disease receiving initial treatment with nivolumab plus AVD chemotherapy (N+AVD) to outcomes for patients receiving brentuximab vedotin plus AVD, a standard combination. AVD stands for the chemotherapy drugs doxorubicin/Adriamycin, vinblastine and dacarbazine.
“This study is unusual because there aren’t many phase 3 studies in which we have a significant cohort of kids along with adults — multiple ages being treated essentially the same way,” said LeBlanc. Researchers enrolled 970 patients, some as young as 12 years old, at 256 sites in the U.S. and Canada. The study chair was Alex Herrera, MD, from City of Hope.
Patients on the investigational therapy had significantly lower risk of disease progression or death compared to those on the standard regimen. Treatment with nivolumab was also better tolerated by patients. After formal interim analysis, the SWOG Data and Safety Monitoring Committee recommended releasing the trial data, which showed positive results, in 2023. Now, a follow-up analysis has confirmed the findings. LeBlanc noted that successful conduct and analyses for the trial would not have been possible without Fred Hutch staff statistical colleague, Hongli Li, MS, also an author on the paper.
Based on the results, N+AVD should be a strong candidate for primary treatment in adolescent and adult populations with stage 3 or 4 Hodgkin lymphoma, according to the published report. Spurred by the promising findings, the maker of nivolumab, Bristol-Myers Squibb, intends to request approval from the U.S. Food and Drug Administration to use nivolumab in this clinical setting.
Like Tangen, LeBlanc champions the role of biostatisticians in careful study design.
“We try to limit the data points we’re bringing in, focusing on the key things we need in order to answer the questions for that study,” he said. “We want to do studies that have impact, so our goal is to keep the data ‘lean and mean.’”
LeBlanc has been with Fred Hutch for 30 years — not as long as the SWOG SDMC has been here, but long enough to have witnessed the center’s evolution.
“One of the accomplishments we’re quite proud of is that we adopted standards across diseases that we’ve used for a long time now, such as how the team codes for disease progression. While ‘progression’ for each disease, such as lymphoma versus leukemia versus melanoma, may have its own definition, progression appears in our database in a standard way. Standardized reporting mechanisms and tools help to ensure that the results of studies are reproducible — that following the same procedures will lead to the same answer,” he said, such as whether a particular treatment regimen is more effective in a certain type of patient and situation.
Teamwork underpins each successful study
Together, the SWOG SDMC at Fred Hutch and CRAB have more than 100 staff members who contribute to SWOG research in a variety of ways. Along with faculty biostatisticians, there are statistical research associates, statistical unit assistants, data coordinators, administrators, applications developers, information technology specialists and more. Both LeBlanc and Tangen stress that when their names appear on published manuscripts in the New England Journal of Medicine or elsewhere, they represent a multitude of staff members who’ve put in hours of meticulous work on both the statistical science and data management of the trials.
“So many people are part of the team,” said Tangen. “I’m a general for a huge army behind me.”
— By Laurie Fronek
The bladder cancer study was supported by grants from the NCI and the Canadian Cancer Society.
The Hodgkin lymphoma study was funded by the NCI, with additional support provided by Bristol-Myers Squibb (BMS) through a cooperative research and development agreement between NCI and BMS. Brentuximab vedotin was provided by Seagen. This research was also supported by the Leukemia and Lymphoma Society, the Lymphoma Research Foundation, the V Foundation for Cancer Research and the Miller Family Fund.
Laurie Fronek is a writer and editor specializing in health and medicine. Based in Seattle, she has written for health care-industry clients, including clinics, hospitals, research institutions, insurers and publishers, around the country. Reach her at lauriefronek@comcast.net.
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org.
Engineered HSV can trigger genetic chain reaction, rejigger HSV genes during co-infection
Proof-of-concept work raises hope that ‘gene drive’ could one day form basis of curative gene therapy for herpes
In preclinical work recently published in Nature Communications, Fred Hutch Cancer Center scientists used a genetic “chain reaction” to transform herpes simplex virus DNA during an HSV infection. The proof-of-concept study, which used a CRISPR gene editing tool to change the color of fluorescent viruses, potentially opens the door to a treatment that uses HSV-based gene therapy to cure HSV.
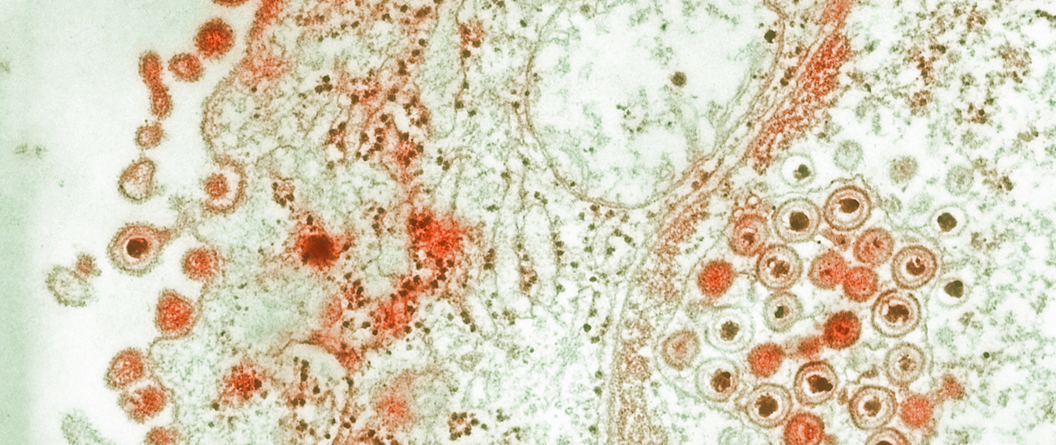
Read More
“This paper is really about establishing that [this genetic strategy] could be something that could work in the future,” said Fred Hutch virologist Marius Walter, PhD, the staff scientist in the Jerome Lab who led the project. “It’s proof of concept for a new technology, but we don’t know yet if it will work and how much it will work.”
Walter capitalized on a phenomenon called “gene drive,” which can push a gene variant through a population, to retool HSV DNA lurking in infected neurons. His strategy also used engineered HSV virions to carry gene editing technology to the neurons where latent HSV DNA hides out.
While Walter didn’t alter HSV infectivity (that’s the subject of future work), he did show that engineered HSV can co-infect neurons with non-engineered HSV, which makes gene drive possible.
An HSV-based gene therapy built off this concept could act as a sheep in wolf’s clothing: a defanged HSV able to infiltrate infected cells and inactivate lurking viral DNA, rendering it toothless.
If successful, it would be a second gene therapy strategy to target HSV developed by Keith Jerome, MD, PhD, and his team
“It’s always good to have multiple shots on goal — because this is a big goal,” Jerome said.
Seeking a cure for an age-old virus
HSV has been infecting humans for six million years. The World Health Organization estimates that nearly 65% of people under age 50 carry HSV, which can cause sores around the lips (usually HSV-1) or genitals (HSV-1 or HSV-2). The virus targets neurons; after an initial infection, it sets up a lifelong, latent infection and HSV DNA remains dormant in the form of DNA circles called episomes.
During the latent phase of infection, the virus can reactivate and produce new virions that travel through the nerves toward the skin, causing herpes outbreaks. Though many people experience no or tolerable symptoms, others find the infection to have debilitating effects on their health and quality of life. In rare cases, HSV can cause meningitis, encephalitis, blindness and, even more rarely, death. Herpes encephalitis is particularly dangerous in newborns who contract HSV from HSV-positive mothers during childbirth.
Herpes symptoms can be managed with antiviral therapy, but there is currently no vaccine or cure. And while the HSV episomes lurking in neurons are a source of new virus, they also pose an attractive target for potentially curative gene therapies.
Jerome’s team has spent years investigating strategies to cure or minimize this latent, lingering HSV DNA in neurons, as a way to minimize or perhaps even cure HSV infections.
“But it’s always good to have other options, more tools,” Jerome said.
Walter had previously developed a gene drive strategy that works in cytomegaloviruses, a member of the larger Herpesviridae virus family to which HSV belongs. Both Walter and Jerome were excited at the prospect of exploring the approach in HSV.
“I always loved the concept of gene drives in Marius’ work when he was at his previous institution, so the opportunity to have him come join us … and move it into model organisms was incredibly exciting,” Jerome said.
Gene drive: a genetic chain reaction
Gene drive enables a genetic trait to spread more rapidly through a population than average. If the “drive” is strong enough, the phenomenon can ensure that nearly all members of that population carry the trait.
“The concept of the gene drive is really just like a chain reaction that gets amplified,” Walter said.
It’s been studied as a way to help control populations of disease-carrying animals. Scientists have tested it in mosquitos as a way to reduce malaria transmission by heavily biasing mosquito sex ratios away from biting females and toward non-biting males.
Though viruses don’t mate like mosquitos do, some species can mingle their genetic information when individual viruses meet inside the same cell. In this case, gene drive could be used to inactivate HSV DNA.
Walter needed to show that gene drive pushes genetic changes through HSV DNA in infected cells. He also wanted to discover whether DNA from different HSV virions would mix in the same neurons during infection.
To do this, he used HSV as the delivery and targeting vehicle for his gene drive strategy.
The bonus to using HSV as the gene drive carrier is that it naturally goes where natural infection occurs, Walter said. (An HSV-based therapeutic already has precedent: earlier this year an oncolytic HSV therapy was fast-tracked by the U.S. Food and Drug Administration to treat head and neck cancer.)
“You’re basically using herpes to target herpes,” he said. “The idea is that it would be super specific and maybe will reach the relevant cells with a high efficiency.”
He set out to answer two questions: would his gene drive strategy work for HSV as it did for CMV, and were conditions during infection conducive to using an HSV-based gene therapy?
Gene drive works during HSV infection
Walter had previously developed a CRISPR-based gene drive method that takes advantage of natural viral DNA mingling (also known as recombination) to insert a gene for a red fluorescent protein into viral DNA. This allows him to see gene drive in action: because the HSV he’s targeting naturally glows yellow, a shift to orange indicates gene drive has occurred.
For the current study, Walter engineered HSV-1 to carry the DNA editing strategy to neurons, where HSV infects and hangs out during latency.
He quickly showed that in lab dishes, engineered and non-engineered HSV virions could easily co-infect the same cells and undergo genetic modification.<
“In a Petri dish, this is easy because you end up having a limited amount of cells and tons of virus, so a lot of the viruses are going to infect the same cells,” Walter said. “The big question that we spend a lot of time in this paper addressing is, ‘How much do you have co-infection happening in vivo?’”
He addressed the question using two scenarios: an acute infection and a reactivated latent infection. In a mouse model of acute HSV-1 encephalitis, he showed that in some areas of the nervous system, as many as 80% of the viruses showed evidence of gene drive.
Walter then used a mouse model of HSV-1 latency and reactivation (HSV doesn’t naturally reactivate in mice, so scientists use a drug to turn it back on). He found several examples of recombination with the non-engineered viruses, showing that both co-infection and gene drive can occur.
“We showed that you often have two viruses that infect the same cell,” Walter said. “This is where the paper brings in a lot of interesting basic biology, because it shows that [co-infection is] actually happening at a high frequency, which nobody had really shown in that way before.”
Next steps: finding a balance
Now that he and Jerome have evidence that gene drive can occur during latent HSV infections, they’re working on moving that insight further, Walter said.
“We’re trying to see if we can use the technique as a way to prevent or cure disease,” he said.
It’s a tricky goal.
“You need an engineered virus that by itself does not cause disease, but needs to be able to meet and recombine with the wild-type virus to inactivate it,” Walter said. “So we need to find the balance between having an engineered virus that in itself doesn’t cause disease, but gets where it needs to go.”
To hopefully discover where that balance lies, Walter is moving his method to a preclinical model that better mimics the course of human HSV infection. He’s also working to address key questions about the factors that influence how efficiently gene drive occurs during latent HSV infection. What Walter learns in models of HSV-1 he will also translate to HSV-2, he said.
Whether gene drive could underpin a therapy that works during HSV dormancy, or would need to be administered during a reactivation phase, will become more clear with more work.
“The goal is to reduce the level of viral shedding and disease, but if we only reduce the level of symptomatic disease, it’s already a big win,” Walter said.
Jerome also noted that insights from Walter’s work complemented the other gene therapy approaches being studied in his lab, and vice versa. The team immediately use lessons learned from one approach to improve the other, he said.
“It’s really sped up the overall work and that’s really the goal here: To provide something new to help people who are living with the virus,” Jerome said.
— By Sabrina Richards
This work was funded by Fred Hutch Cancer Center, the National Institutes of Health and the Buck Institute for Aging Research.
Sabrina Richards, a staff writer at Fred Hutchinson Cancer Center, has written about scientific research and the environment for The Scientist and OnEarth Magazine. She has a PhD in immunology from the University of Washington, an MA in journalism and an advanced certificate from the Science, Health and Environmental Reporting Program at New York University. Reach her at srichar2@fredhutch.org.
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org.
Around the Earth and back: Obliteride unites thousands to rack up miles, raise $9M to cure cancer faster
Annual bike ride and 5K walk/run surpasses previous participation and fundraising records with 6,260 participants, 814 volunteers and more than 22,000 donors.

On Saturday, August 10, more people than ever before experienced cheers, sun and community as they joined up in Seattle and virtually for Obliteride, Fred Hutch Cancer Center’s annual bike ride, 5K walk/run and fundraiser. Together, they biked, ran and walked more than 98,000 miles — the distance it would take to circle the Earth almost four times! — to help cure cancer faster.
Read more
Obliteride’s impact
Philanthropic support from Obliteriders and other generous donors provides crucial funding for transformative discovery at Fred Hutch.
“This passionate community has a profound impact,” said Kelly O’Brien, vice president of philanthropy. “We are honored by their partnership and dedication. Obliteride support helps us accelerate the pace and scale of research, hire and retain leading scientists and continue to shorten the distance from laboratory to clinic.”
O’Brien emphasized that Obliteride fundraising also supports early-stage research, potentially unlocking millions in federal and foundation funding once projects are off the ground.
Many Obliteride participants share a personal commitment to helping Fred Hutch speed the pace and scale of research. Obliteride offers them the opportunity to fundraise for an area of work at Fred Hutch that is the most meaningful to them, including a specific disease area, program, researcher or clinician. Funds raised by Obliteride’s 2024 season will fuel research for 27 disease areas, 86 faculty members and 25 programs and specialty areas at Fred Hutch.
 Bike rider Alan Schulkin and his team, Equipe Rouge, chose to fundraise for Fred Hutch’s general fund for top research and care priorities.
Bike rider Alan Schulkin and his team, Equipe Rouge, chose to fundraise for Fred Hutch’s general fund for top research and care priorities.
“Curing cancer is personal to me, having survived three cancers [testicular cancer, leukemia and bladder cancer] over the last 24 years,” said Schulkin, who is a 12-year participant. “I want everyone to have the chances I’ve had to benefit from the cutting-edge research taking place at Fred Hutch.”
To learn more about Obliteride’s powerful impact and community, explore the Obliteride 2024 Annual Report.
A powerful and personal event
Obliteride unites thousands of people who, like Schulkin, have or have had cancer, along with friends and family members, Fred Hutch clinicians and staff and all those passionate about cures.
Second-year rider Martha Lane fundraised for Fred Hutch’s lung cancer research.
“It has been a bit of a wild ride, as my treatment for stage 4 non-small cell lung cancer is ongoing,” said Lane, who was also a top 2024 fundraiser. She described her 25-mile route at Obliteride as a different “wild ride” — one she completed on her beloved bicycle, “Hope.”
Jonathan G. Sham, MD, MBEE, a surgical oncologist at Fred Hutch and UW Medicine, shared remarks with the crowd at the 5K walk/run starting line. He emphasized that Obliteriders are partners in the work of scientific innovation.
“Over the past 10 years, we’ve made huge advances,” he said. “But those advances didn’t happen on their own. They took grit, dedication and action. That is what we’re doing together: taking action.”
Dr. Sham completed the walk/run with his team, which included friends, family and several of his patients.

Obliteride welcomes new leader
In October, Obliteride welcomed Tracy Evans as senior executive director. Using her deep experience in peer-to-peer and athletic fundraising events, Evans will build on Obliteride’s momentum to expand the event.
Evans rode the 50-mile bike route at Obliteride 2024. “The energy of the weekend was amazing, as our participants supported one another and raised funds to help obliterate cancer,” she said. “It was a wonderful experience to be with this energized and powerful community!”
Evans said that preparations are already underway for Obliteride’s 2025 season.
“Together, this outstanding community is taking action to fund world-changing research, help Fred Hutch advance transformative discoveries and improve cancer prevention, detection, treatment and care,” said Evans. “We invite everyone to join us next August as we reach even higher to help cure cancer faster!”
— By Laura Anderson
Save the date for next year’s Fred Hutch Obliteride
Saturday, August 9, 2025
All are invited to bike ride, walk, run or volunteer and help cure cancer faster.
Are you interested in reprinting or republishing this story? Be our guest! We want to help connect people with the information they need. We just ask that you link back to the original article, preserve the author’s byline and refrain from making edits that alter the original context. Questions? Email us at communications@fredhutch.org.
Laura Anderson is a philanthropy writer for Fred Hutch Cancer Center. She draws on her background in philanthropy, publishing and global health communication to share the power of research and the stories of those who support it. In addition to positions at the Seattle-based global health nonprofit PATH, the University of Washington Department of Global Health, and Holt, Rinehart & Winston in New York, she has worked as a travel planner, an animal mascot and as a laborer on the Appalachian Trail. Reach her at: laander4@fredhutch.org.
Join the Thomas Legacy Society
The Thomas Legacy Society at Fred Hutch is a special group of supporters who have made a commitment through a legacy gift to drive lifesaving cancer research and improve the lives of patients in the years to come.
What does a unique gift like this actually do? By making a gift to Fred Hutch in your will or trust, or by naming us as beneficiary on one of your financial or investment accounts, you join a community fueling innovation by:
- Funding early-stage ideas that push the boundaries of science and are not yet eligible for federal funding.
- Discovering new ways to prevent diseases from ever occurring.
- Supporting early-career investigators who are working on the cures of the future.
- Investing in technology and equipment that accelerate discovery.
- Expanding support services for patients and caregivers.
Visit our website to learn more or contact us.
Have you already included Fred Hutch in your estate plans? Please let us know so we can ensure your wishes are carried out as you intend!

As we mark our 50th anniversary in 2025, Fred Hutch is looking beyond what’s possible today to a new era of discovery. With your partnership, our Campaign for Fred Hutch will transform the pace and scale of innovation so we can redefine cancer and infectious disease for generations to come.
